if I Collect Your Blood
You lot are here: Geisinger Medical Laboratories > Specimen Drove Transmission and Examination Itemize > Blood Specimen Drove and Processing
Blood Specimen Collection and Processing
The first step in acquiring a quality lab test outcome for any patient is the specimen collection procedure. The venipuncture procedure is complex, requiring both knowledge and skill to perform. Several essential steps are required for every successful collection process:
Venipuncture Process:
- A phlebotomist must have a professional, courteous, and understanding mode in all contact with all patients.
- The first step to the collection is to positively place the patient by two forms of identification; inquire the patient to state and spell his/her name and give you his/her nativity engagement. Cheque these against the requisition (paper or electronic).
- Cheque the requisition form for requested tests, other patient information and whatever special draw requirements. Gather the tubes and supplies that you will need for the draw.
- Position the patient in a chair, or sitting or lying on a bed.
- Wash your hands.
- Select a suitable site for venipuncture, by placing the tourniquet 3 to 4 inches above the selected puncture site on the patient. See below for venipuncture site selection "notes."
- Practise not put the tourniquet on too tightly or get out it on the patient longer than 1 minute.
- Next, put on not-latex gloves, and palpate for a vein.
- When a vein is selected, cleanse the area in a circular motility, starting time at the site and working outward. Permit the area to air dry out. After the area is cleansed, information technology should not be touched or palpated again. If you find it necessary to reevaluate the site by palpation, the surface area needs to be re-cleansed before the venipuncture is performed.
- Ask the patient to make a fist; avert "pumping the fist." Grasp the patient's arm firmly using your thumb to draw the skin taut and anchor the vein. Swiftly insert the needle through the skin into the lumen of the vein. The needle should form a 15-30 degree bending with the arm surface. Avert backlog probing.
- When the last tube is filling, remove the tourniquet.
- Remove the needle from the patient's arm using a swift astern movement.
- Place gauze immediately on the puncture site. Apply and hold adequate pressure level to avoid formation of a hematoma. Afterwards property pressure level for 1-2 minutes, record a fresh piece of gauze or Band-Aid to the puncture site.
- Dispose of contaminated materials/supplies in designated containers.
Notation: The larger median cubital and cephalic veins are the usual choice, but the basilic vein on the back of the arm or dorsal hand veins are too acceptable. Foot veins are a last resort because of the college probability of complications.
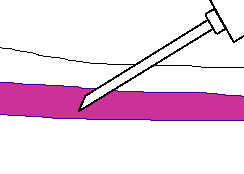
Fingerstick Process:
- Follow steps #one through #five of the procedure for venipuncture as outlined higher up.
- The best locations for fingersticks are the 3rd (eye) and 4th (band) fingers of the non-dominant mitt. Practice not use the tip of the finger or the eye of the finger. Avoid the side of the finger where there is less soft tissue, where vessels and nerves are located, and where the os is closer to the surface. The 2nd (index) finger tends to have thicker, callused pare. The 5th finger tends to have less soft tissue overlying the bone. Avert puncturing a finger that is cold or cyanotic, swollen, scarred, or covered with a rash.
- When a site is selected, put on gloves, and cleanse the selected puncture area.
- Massage the finger toward the selected site prior to the puncture.
- Using a sterile safety lancet, make a pare puncture just off the center of the finger pad. The puncture should be made perpendicular to the ridges of the fingerprint and so that the drib of claret does not run down the ridges.
- Wipe away the offset drop of blood, which tends to contain backlog tissue fluid.
- Collect drops of claret into the collection tube/device by gentle pressure level on the finger. Avoid excessive pressure or "milking" that may clasp tissue fluid into the drop of claret.
- Cap, rotate and capsize the drove device to mix the blood nerveless.
- Have the patient agree a small gauze pad over the puncture site for a few minutes to cease the bleeding.
- Dispose of contaminated materials/supplies in designated containers.
- Label all appropriate tubes at the patient bedside.
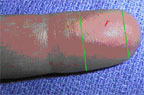
Heelstick Process (infants):
The recommended location for blood drove on a newborn baby or infant is the heel. The diagram below indicates the proper area to use for heel punctures for claret collection.
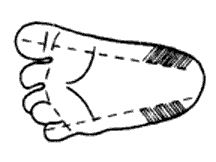
- Prewarming the infant's heel (42° C for 3 to 5 minutes) is important to increase the flow of blood for drove.
- Wash your hands, and put gloves on. Clean the site to be punctured with an alcohol sponge. Dry the cleaned area with a dry out gauze pad.
- Agree the baby's foot firmly to avoid sudden motility.
- Using a sterile claret condom lancet, puncture the side of the heel in the appropriate regions shown above. Make the cut across the heel impress lines so that a drop of claret tin can well up and not run downwardly along the lines.
- Wipe away the first drop of blood with a slice of make clean, dry cotton gauze. Since newborns do non often bleed immediately, use gentle force per unit area to produce a rounded drop of claret. Do not utilize excessive pressure because the blood may get diluted with tissue fluid.
- Fill the required microtainer(s) equally needed.
- When finished, elevate the heel, place a slice of clean, dry cotton on the puncture site, and hold information technology in identify until the bleeding has stopped. Apply record or Rough-and-tumble to area if needed.
- Exist sure to dispose of the lancet in the appropriate sharps container. Dispose of contaminated materials in advisable waste receptacles.
- Remove your gloves and wash your hands.
Order of Draw:
Blood collection tubes must exist drawn in a specific guild to avert cross-contamination of additives betwixt tubes. The recommended order of describe is:
- Commencement - blood civilization bottle or tube (yellow or yellow-black top)
- 2nd - coagulation tube (light blue top).
- Tertiary - not-additive tube (red superlative)
- Last draw - condiment tubes in this order:
- SST (red-grayness or gilt peak). Contains a gel separator and clot activator.
- Sodium heparin (dark green acme)
- PST (night green green top with gold rim). Contains lithium heparin anticoagulant and a gel separator.
- EDTA (lavender summit)
- Oxalate/fluoride (lite grayness tiptop) or other additives
NOTE: Tubes with additives must be thoroughly mixed. Clotting or erroneous test results may be obtained when the claret is not thoroughly mixed with the additive.
Labeling The Sample
All specimens must exist received by the laboratory with a legible characterization containing at to the lowest degree two (ii) unique identifiers.
The specimen must be labeled with the patient's full name (preferably last name first, and so outset name last) and one of the following:
- Geisinger medical record number (MRN) - for Geisinger locations, this is the required second identifier
- Patient'due south full date of nascence (must include the month, day, and year)
- Unique requisition identifier/label
Areas to Avoid When Choosing a Site for Blood Draw:
Sure areas are to be avoided when choosing a site for blood draw:
- All-encompassing scars from burns and surgery - it is difficult to puncture the scar tissue and obtain a specimen.
- The upper extremity on the side of a previous mastectomy - test results may be affected because of lymphedema.
- Hematoma - may cause erroneous test results. If another site is not available, collect the specimen distal to the hematoma.
- Intravenous therapy (Four) / claret transfusions - fluid may dilute the specimen, and so collect from the reverse arm if possible.
- Cannula/fistula/heparin lock - hospitals have special policies regarding these devices. In full general, claret should not be drawn from an arm with a fistula or cannula without consulting the attending doc.
- Edematous extremities - tissue fluid accumulation alters test results.
Techniques to Forestall Hemolysis (which can interfere with many tests):
- Mix all tubes with anticoagulant additives gently (vigorous shaking tin can crusade hemolysis) 5-10 times.
- Avert drawing blood from a hematoma; select another draw site.
- If using a needle and syringe, avert cartoon the plunger back as well forcefully.
- Brand certain the venipuncture site is dry before proceeding with describe.
- Avoid a probing, traumatic venipuncture.
- Avert prolonged tourniquet application (no more than 2 minutes; less than 1 minute is optimal).
- Avert massaging, squeezing, or probing a site.
- Avoid excessive fist clenching.
- If blood flow into tube slows, adjust needle position to remain in the center of the lumen.
Claret Sample Handling and Processing:
Pre-centrifugation Handling - The first critical step in the lab testing process, after obtaining the sample, is the preparation of the claret samples. Specimen integrity tin be maintained by following some basic treatment processes:
- Fill up tubes to the stated draw book to ensure the proper blood-to-additive ratio. Allow the tubes to fill until the vacuum is exhausted and claret flow ceases.
- Tubes should be stored at 4-25°C (39-77°F).
- Tubes should not be used across the designated expiration date.
- Mix all gel barrier and additive tubes by gentle inversion 5 to10 times immediately afterward the describe. This assists in the clotting process. This also assures homogenous mixing of the additives with the blood in all types of additive tubes.
- Serum separator tubes should jell for a full xxx minutes in a vertical position prior to centrifugation. Short clotting times can result in fibrin formation, which may interfere with complete gel barrier formation.
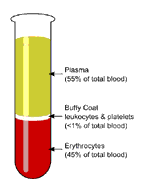
Blood Sample Centrifugation – Information technology is recommended that serum be physically separated from contact with cells every bit soon as possible, with a maximum time limit of two hours from the time of drove.
- Complete gel bulwark germination (gel bulwark tubes) is time, temperature and G-force dependent. The uniformity of the barrier is time dependent; an incomplete barrier could issue from shortened centrifugation times.
- In general, for a horizontal, swing-saucepan centrifuge, the recommended spin time is 10 minutes. For a fixed-bending centrifuge, the recommended spin time is xv minutes.
- Annotation: Gel menstruation may exist impeded if chilled before or after centrifugation.
- Tubes should remain closed at all times during the centrifugation process.
- Place the airtight tubes in the centrifuge as a "balanced load" noting the post-obit:
- Opposing tube holders must exist identical and contain the aforementioned cushion or none at all.
- Opposing tube holders must be empty or loaded with equally weighted samples (tubes of the same size and equal in make full).
- If an odd number of samples is to be spun, fill a tube with water to match the weight of the unpaired sample and place it across from this sample.
Centrifuge Safety
- Interference with an activated centrifuge past an impatient employee can result in bodily injury in the form of directly trauma or aerosolization of hazardous aerosol.
- Centrifuges must never be operated without a cover in place.
- Uncovered specimen tubes must not be centrifuged.
- Centrifuges must never be slowed downwardly or stopped past grasping part(s) of the device with your mitt or by applying another object against the rotating equipment.
- Be sure the centrifuge is accordingly balanced earlier activating. If an abnormal racket, vibration, or audio is noted while the centrifuge is in performance, immediately stop the unit (plough off the switch) and check for a possible load imbalance.
- Clean the centrifuge daily with a disinfectant and newspaper towel. Broken tubes or liquid spills must be cleaned immediately.
pellerinhaded1983.blogspot.com
Source: https://www.geisingermedicallabs.com/catalog/blood_specimens.shtml
0 Response to "if I Collect Your Blood"
Postar um comentário